PEM Fuel Cell Analysis
This was the final partner project for Intermediate Thermodynamics. Using the Cantera toolbox in MATLAB, we were to investigate a proton-exchange membrane (PEM) fuel cell while varying current density, temperature, and ambient pressure.
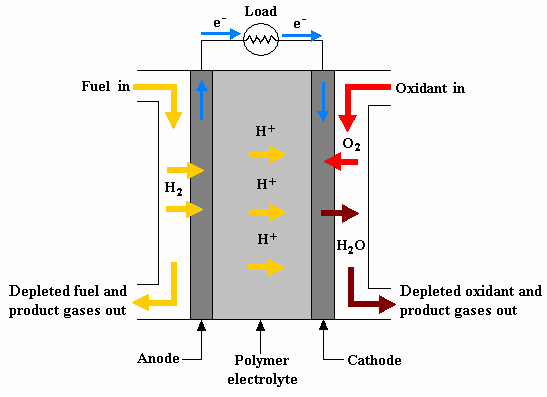
Overview
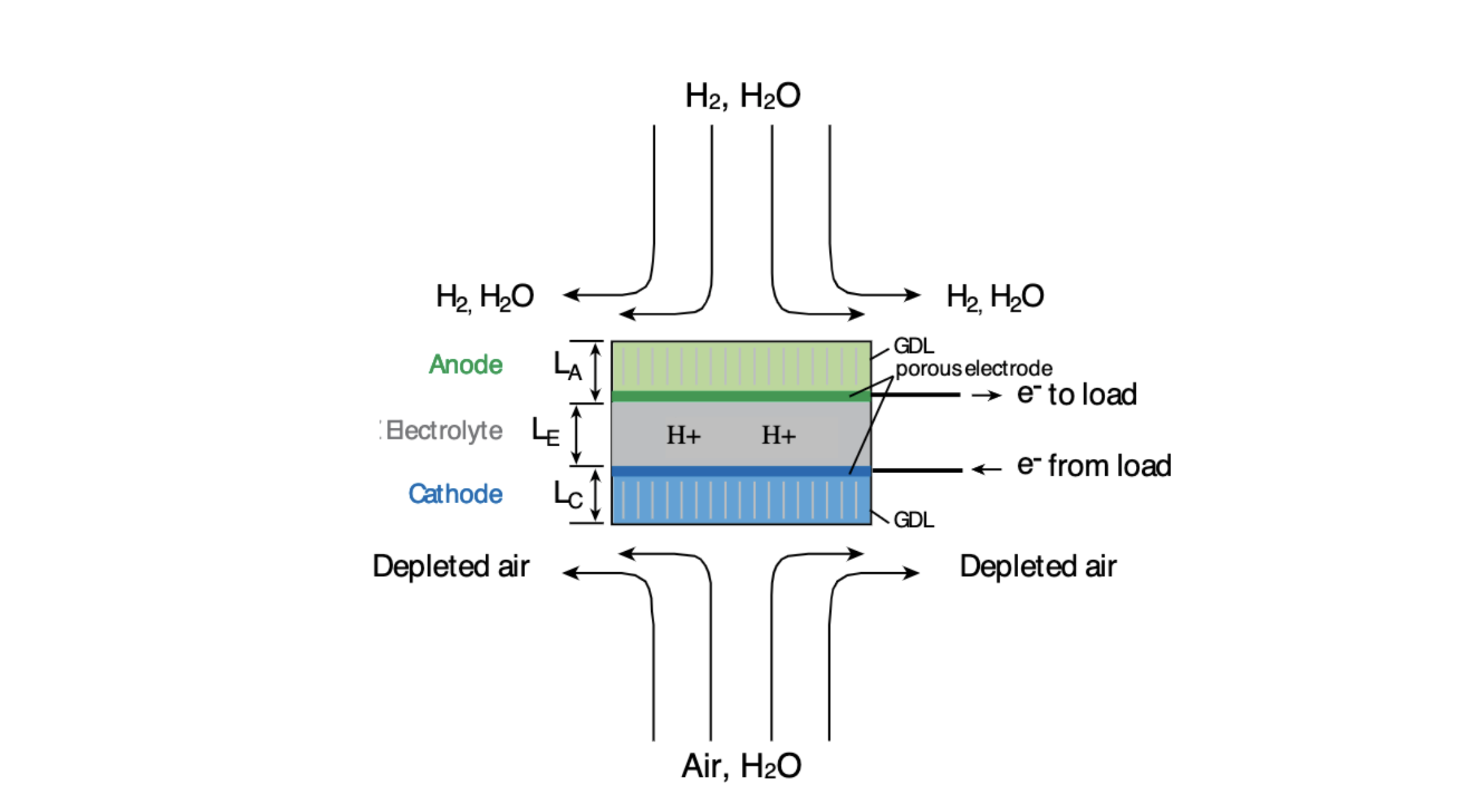
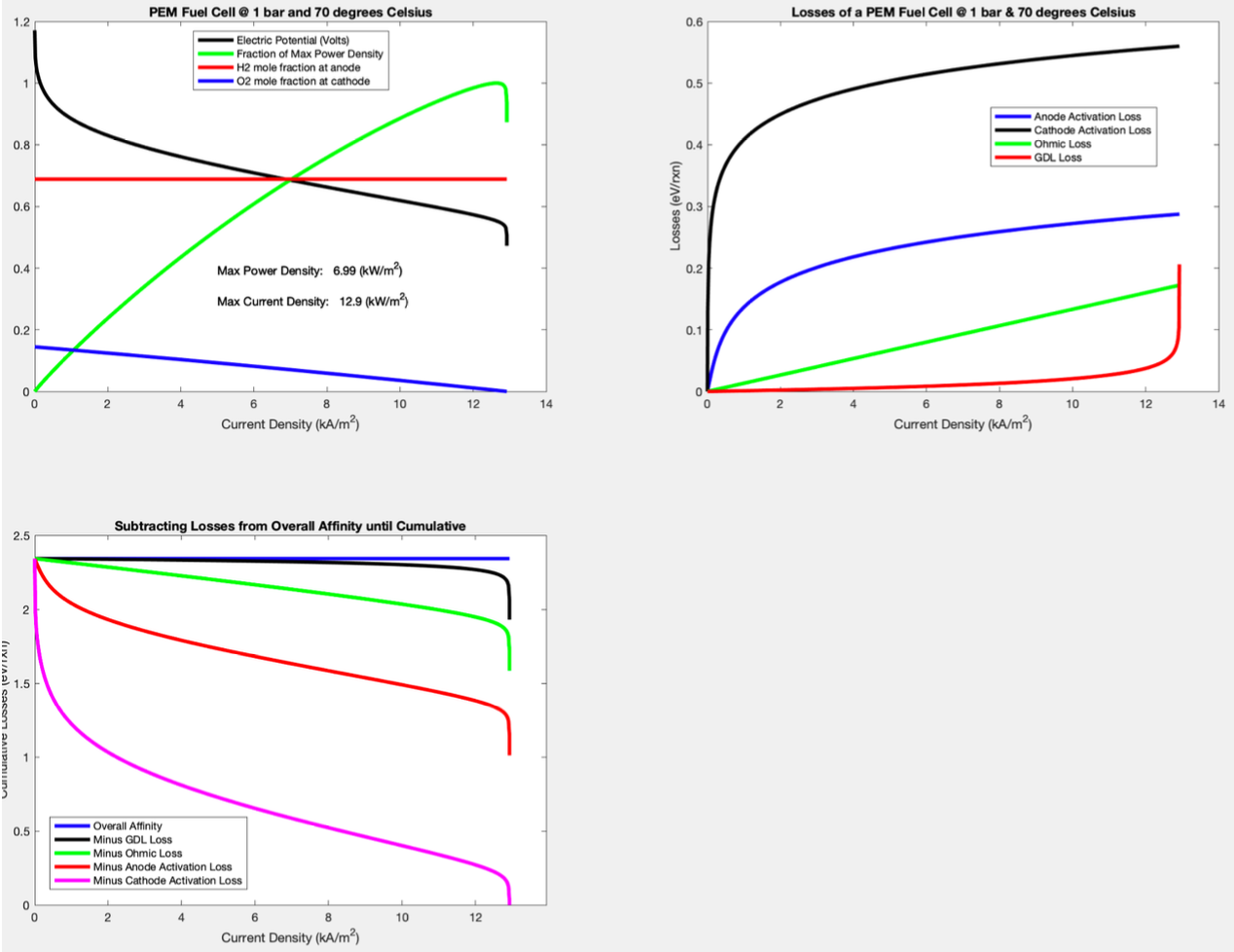
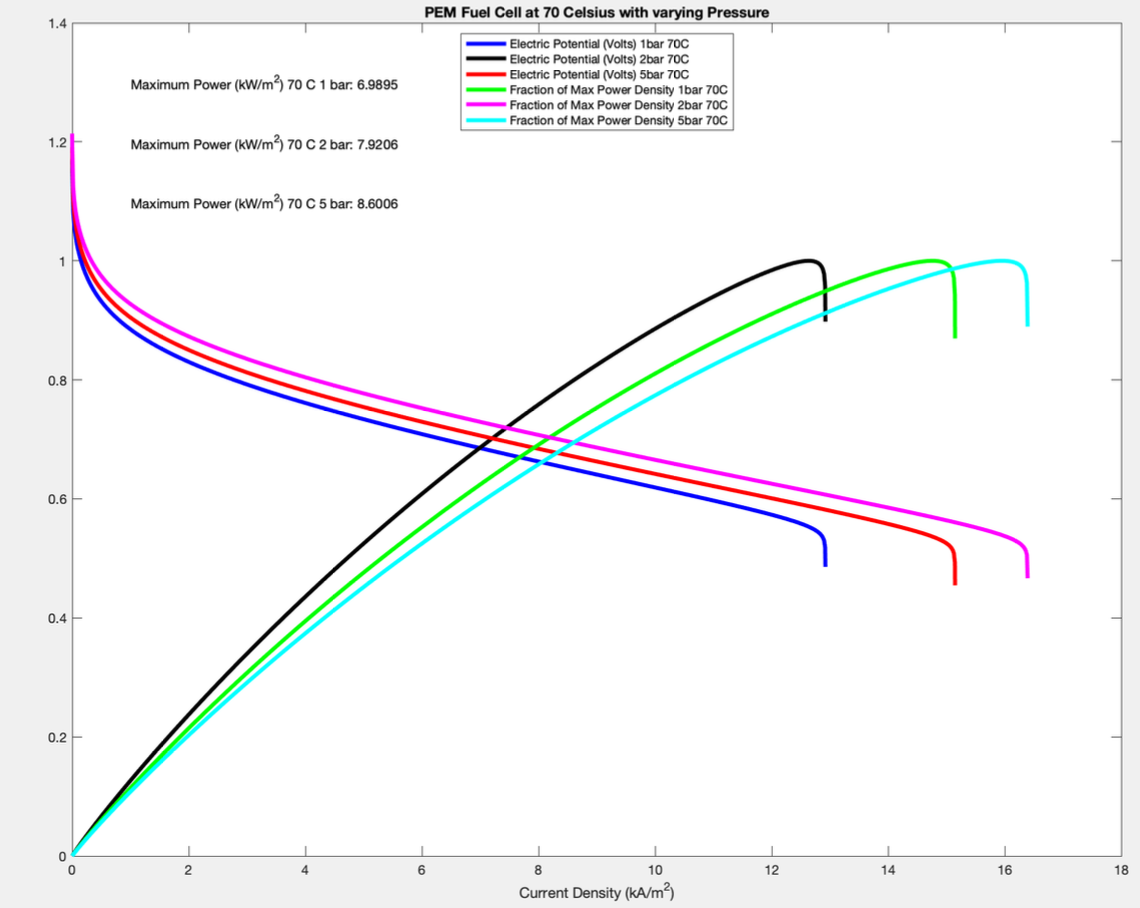
Given the geometry and a few assumptions, we were to first find the affinity and GDL, Ohmic, and activation losses of a button cell in a PEM Fuel cell in equilibrium given a set of conditions (1 bar, 70 degrees C) while varying current density. Then we were to find the optimal current density at different operating temperatures and pressures.
Process
First, we found the initial states and created gas objects to represent the conditions at the anode and cathode using the Cantera toolbox. Then we used the stoichiometric relationships between the chemical and electrochemical potentials of each contributing constituant at the anode and cathode to find the electrochemical potential (ECP) of the electrons at the electrodes. We used the difference between the electron ECPs to find the affinity of the button cell. We then found the losses using electric relationships and then graphed them.
Assumptions and Givens
Temperature = 70 degrees Celsius
Pressure = 100,000 Pascals
Conductivity = 15 S/m
Thickness of the cathode and electrode = 0.2 mm
Length of the gas diffusion layer (GDL) = 5 mm